Warning signs: Bill would provide obstacle in mass tort cases
By: Jack Zemlicka, [email protected]//October 21, 2011//
Warning signs: Bill would provide obstacle in mass tort cases
By: Jack Zemlicka, [email protected]//October 21, 2011//
Attorney Kevin Martin isn’t looking forward to telling a female client with shards of a medical implant imbedded in her bladder that she can’t sue the maker because her doctor didn’t have to tell her about the risks.
But Martin, of Brookfield-based Cannon & Dunphy SC, said that’s a conversation he’ll have if a state bill passes, making drug companies and other manufacturers immune from having to warn physicians of dangerous side effects. U.S. Food and Drug Administration labels still would be required on the products.
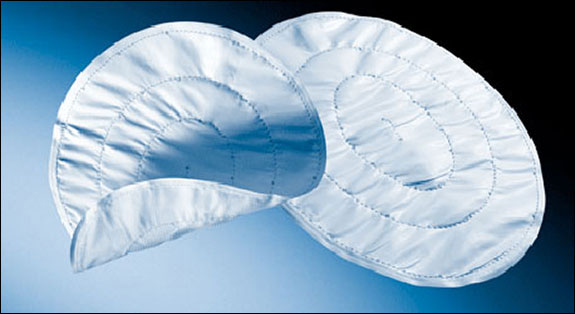
The proposal from Gov. Scott Walker and other Republican legislators comes at a time when plaintiffs’ lawyers are evaluating claims against 26 companies that produced a product designed to relieve organ pressure in women suffering from incontinence. The product, called a transvaginal mesh, was not approved by the FDA.
“Could this potentially eliminate a client’s right to recover all together?” Martin said. “Yes.”
The mesh is a hammock-shaped, petroleum-based implant that is sewn into tissue and prevents the bladder or other organs from putting pressure on the vagina.
According to the FDA, the number of adverse event reports tied to transvaginal mesh has risen from 303 in 2008 to 620 last year.
Injuries, Martin said, arise from the tissue attached to the device, and the device itself, eroding. That erosion can cause infection, nerve damage and internal bleeding, he said.
There is one federal multidistrict litigation case pending in West Virginia involving 126 lawsuits, but none have been filed in Wisconsin.
That will change, said Jason Abraham, an attorney with Milwaukee-based Hupy & Abraham SC. But absent a failure-to-warn claim, some attorneys might shy away from even taking the cases.
“Some cases, attorneys just won’t be interested in anymore,” he said. “It’s not going to stop me right now from looking into them, but it’s going to come down to a conversation with a potential client as to if we have a case.”
A failure-to-warn claim is one of three, along with improper manufacturing and improper design, traditionally made in mass tort cases involving defective products.
Martin said juries can deny claims that the maker didn’t design or build the product properly, leaving only a failure-to-warn claim.
“One of the things I’m asked is: ‘Can’t your client get money through another potential claim?’” Martin said. “The answer is potentially no.”
Failure to warn was a significant claim in successful cases brought against drug maker Merck & Co. for the painkiller Vioxx. Use of the drug allegedly doubled the risk of heart attacks and strokes, a warning not on the label of the drug.
The company recalled the drug in 2004 and agreed to a $4.85 billion settlement in 2007. Last year, the company completed payment of more than 3,400 claims to families of Vioxx users who died of heart attacks or strokes.
“If this bill passes as is,” Martin said, “Merck and Vioxx would have been immune of any failure-to-warn claims.”
Defense attorneys involved in transvaginal cases declined to comment on the affect the proposed legislation would have on cases.
Cullen Werwie, Walker’s press secretary, said the goal of the bill, as part of the Back to Work Wisconsin Special Session, is to create certainty for employers when it comes to liability.
He declined to elaborate on whether the legislation will bar legitimate claims, saying only that, “the tort reform proposals deal directly to the types of claims that have an effect on job creators.”
Abraham said it would be hard to assess the full affect until the legislation passes. Although, he said, if it is retroactive to other mass tort claims, he would have to reconsider cases filed using the failure-to-warn claim.
“Unfortunately, I foresee us having to refile hundreds of lawsuits for current cases we have,” Abraham said, “to make sure the new legislation wouldn’t apply to them.”
Martin said in the next few weeks his firm will file claims in a mass tort case against DePuy Orthopedics Inc., the manufacturer of a defective hip implant, and he said he expects failure to warn will be one of the claims.
He acknowledged it wasn’t the only option for recovery in those cases or in the transvaginal mesh cases, but it often is the most emotionally compelling argument to make to jurors.
“I’ve talked with many jurors after malpractice cases about negligence claims and informed-consent claims,” he said. “Jurors uniformly tell me that, ‘Negligence is a serious issue, but how dare you not tell me what I have the right to now.’
“They take that very personally.”
Legal News
- Wisconsin joins Feds, dozens of states to hold airlines accountable for bad behavior
- Trump ahead of Biden in new Marquette poll
- Bankruptcy court approves Milwaukee Marriott Downtown ‘business as usual’ motion
- New Crime Gun Intelligence Center to launch in Chicago
- Arrest warrant issued for Minocqua Brewing owner who filed Lawsuit against Town of Minocqua
- Wisconsin Supreme Court justices question how much power Legislature should have
- Reinhart named the 2024 Wisconsin law firm of the year by benchmark litigation
- Milwaukee’s Common Council now has the most African Americans, women and openly LGBTQ members ever
- Office of School Safety Provides Behavioral and Threat Assessment Management Training Ahead of 25th Anniversary of Columbine Shooting
- Wisconsin Supreme Court to hear arguments in Democratic governor’s suit against GOP-led Legislature
- Lawsuit asks Wisconsin Supreme Court to strike down governor’s 400-year veto
- Wisconsin man pleads not guilty to neglect in disappearance of boy
WLJ People
- Power 30 Personal Injury Attorneys – Russell Nicolet
- Power 30 Personal Injury Attorneys – Benjamin Nicolet
- Power 30 Personal Injury Attorneys – Dustin T. Woehl
- Power 30 Personal Injury Attorneys – Katherine Metzger
- Power 30 Personal Injury Attorneys – Joseph Ryan
- Power 30 Personal Injury Attorneys – James M. Ryan
- Power 30 Personal Injury Attorneys – Dana Wachs
- Power 30 Personal Injury Attorneys – Mark L. Thomsen
- Power 30 Personal Injury Attorneys – Matthew Lein
- Power 30 Personal Injury Attorneys – Jeffrey A. Pitman
- Power 30 Personal Injury Attorneys – William Pemberton
- Power 30 Personal Injury Attorneys – Howard S. Sicula