Federal Circuit decides human genes are patentable
By: DOLAN MEDIA NEWSWIRES//September 14, 2011//
By Al Turco
Dolan Media
 Intellectual property lawyers say the biotech field dodged a bullet with a recent ruling by the U.S. Court of Appeals for the Federal Circuit finding human genes are patentable.
Intellectual property lawyers say the biotech field dodged a bullet with a recent ruling by the U.S. Court of Appeals for the Federal Circuit finding human genes are patentable.
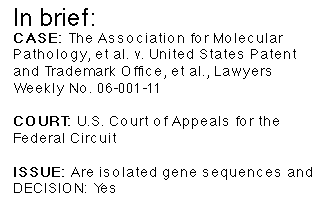
“A decision saying you can’t patent genes would destroy the industry,” Boston lawyer David Resnick said of the closely watched case: The Association for Molecular Pathology, et al. v. United States Patent and Trademark Office, et al.
In its reversal of a lower court judge’s ruling, the Federal Circuit held that isolated human genes are eligible for patents and that methods of isolating and using the genes can also be patentable as long as those methods involve physical steps.
The ruling — referred to as Myriad based on the name of the lead defendant in the case, Myriad Genetics Inc. — means biotech companies can continue to patent gene sequences and the related methods used to predict and prevent disease.
The full text of the 105-page decision, can be ordered at masslawyersweekly.com.
‘Incentivizing innovation’
Resnick, a patent attorney at Nixon Peabody, said among the reasons Myriad is considered “a big deal” is because it involved the exclusive provider of one of the first diagnostic tests for breast and ovarian cancer.
Myriad Genetics owns and aggressively enforces the patents on two gene sequences essential to those tests. Because the tests are costly, there are women who cannot undergo them, Resnick said, noting that several health care interests and the American Civil Liberties Union joined forces to challenge the patents.
 “But the patent system is supposed to incentivize innovation,” he said, adding that biotech companies spend tens of millions of dollars validating the safety and effectiveness of diagnostic tests based on isolated genes before the tests can be used.
“But the patent system is supposed to incentivize innovation,” he said, adding that biotech companies spend tens of millions of dollars validating the safety and effectiveness of diagnostic tests based on isolated genes before the tests can be used.
James Cullem, general counsel of Enzymatics, a biotechnology company in Beverly and a former biotech CEO, said Myriad could be viewed as “much ado about nothing” since the law did not change. But if it had, Cullem said, “the advent of personalized medicine” could have been delayed.
“Isolating genes to get diagnostic tools to help people is what patent law should encourage and protect,” he said.
Cullem acknowledged a “tension behind the scenes” when dealing with human genes in the health care context. “There is always going to be the question of, ‘Is this something we want to protect with a patent or keep as free to all?’”
Lana Gladstein, a partner at Pepper Hamilton in Boston who participated in a recent roundtable discussion of Myriad, predicted that applications for new gene-related biotech patents will be successful if the patent claims are drafted properly.
It is essential for gene patents to specify that the genes are “isolated,” meaning that the sequence for which a patent is sought has been removed from the DNA, said Gladstein, who practices IP litigation.
For method patents, the key is to describe “physical steps,” such as growing new cells, as part of a diagnostic process, she said.
Although such tips will be helpful to clients for now, Gladstein said she expects the Federal Circuit will not be the last court or branch of government to address patents for genes.
“It’s highly likely that the Supreme Court will take up [Myriad] to refine the reasoning of the Federal Circuit,” Gladstein said. “And it may be something that Congress has to legislate.”
Building blocks of life
On May 12, 2009, a coalition of patients, health care providers and researchers represented by the ACLU and the Public Patent Foundation filed suit in U.S. District Court for the Southern District of New York against Myriad Genetics, the U.S. Patent and Trademark Office and the University of Utah Research Foundation.
In short, the plaintiffs challenged Myriad’s patent claims on the isolated human genes BRAC1 and BRAC2; on diagnostic methods for using those genes to test for an increased risk of breast and ovarian cancer in women; and on a screening method to evaluate the potential effectiveness of various therapies.
The District Court judge found the plaintiffs had standing and could proceed with their suit, and on April 19, 2010, the judge invalidated Myriad’s patents regarding BRAC1 and BRAC2 and all the related methods.
Myriad appealed, challenging the plaintiffs’ standing and the invalidation of the patents.
The Federal Circuit subsequently ruled that only one researcher among the plaintiffs had standing.
The court also reinstated Myriad’s patents on BRAC1 and BRAC2 and on the screening method.
The two sides are seeking an en banc rehearing before the Federal Circuit.
Not patently different
In 1874, the Supreme Court held that purification of a natural compound, without more, is not enough to render a product of nature patentable.
More than 100 years later, in 1980, the court carved out three general exceptions to what is patentable under the otherwise broad purview of the U.S. Patent Act: laws of nature, physical phenomena and abstract ideas.
IP attorneys say the courts consistently considered genes patentable until U.S. District Court Judge Robert Sweet’s holding in Myriad.
Combining the physical phenomena exception and the 19th century purification reasoning, Sweet characterized Myriad’s isolated genes as purified natural compounds. Myriad’s BRAC1, BRAC2 and method patents should be invalidated, he said, because the company had done nothing new, but rather had simply observed and compared existing matter and employed the basic scientific method.
Back to the future
Writing for the majority, Judge Alan Lourie said isolated genes are patentable because they are “markedly different — have distinctive chemical identity and nature — from molecules that exist in nature.”
Judge Kimberly Moore concurred, saying isolated genes are patentable because their uses are different from the use of genes found in nature.
But in a dissenting opinion, Judge William Bryson wrote that an isolated gene is “not materially different from the native gene” and compared the process of isolating genes to “snapping a leaf from a tree.”
The judges agreed, however, that Myriad’s screening process, which involved growing new molecules, was patentable because the method amounted to “more than the abstract mental step” of analysis or comparison.
The court also found that one plaintiff — a doctor who had been ready, willing and able to administer one of Myriad’s tests but for concerns of patent infringement — had established standing.
Legal News
- Gov. Evers seeks applicants for Dane County Circuit Court
- Milwaukee man charged in dismemberment death pleads not guilty
- Democratic-led states lead ban on the book ban
- UW Madison Professor: America’s child care crisis is holding back moms without college degrees
- History made in Trump New York trial opening statements
- Prosecutor won’t bring charges against Wisconsin lawmaker over fundraising scheme
- Republican Wisconsin Senate candidate says he doesn’t oppose elderly people voting
- Vice President Harris to reveal final rules mandating minimum standards for nursing home staffing
- Election workers fear threats to their safety as November nears
- Former law enforcement praise state’s response brief in Steven Avery case
- Eric Toney announces re-election bid for Fond du Lac County District Attorney
- Former Wisconsin Democratic Rep. Peter Barca announces new bid for Congress
WLJ People
- Power 30 Personal Injury Attorneys – Russell Nicolet
- Power 30 Personal Injury Attorneys – Benjamin Nicolet
- Power 30 Personal Injury Attorneys – Dustin T. Woehl
- Power 30 Personal Injury Attorneys – Katherine Metzger
- Power 30 Personal Injury Attorneys – Joseph Ryan
- Power 30 Personal Injury Attorneys – James M. Ryan
- Power 30 Personal Injury Attorneys – Dana Wachs
- Power 30 Personal Injury Attorneys – Mark L. Thomsen
- Power 30 Personal Injury Attorneys – Matthew Lein
- Power 30 Personal Injury Attorneys – Jeffrey A. Pitman
- Power 30 Personal Injury Attorneys – William Pemberton
- Power 30 Personal Injury Attorneys – Howard S. Sicula











